Water Quality Indicators: Nutrients
A variety of chemical elements and compounds are essential to the growth and survival of living organisms. In aquatic ecosystems, nitrogen and phosphorus are the most important, as they are most often in short supply relative to the needs of plants, algae, and microbes. Other elements, like iron, manganese, and copper, are needed in small amounts.
Forms of Nutrients
In aquatic ecosystems, nitrogen and phosphorus are found in both particulate and dissolved phases, and in varying chemical forms:
Organic particulate nutrients include living and dead organic matter such as bacterial, plant, and animal tissue.
Inorganic particulate nutrients include minerals and nutrients adsorbed (attracted to the surface) to suspended inorganic sediment particles.
Dissolved organic nutrients include numerous types of biological molecules, such as proteins, containing nitrogen and phosphorus.
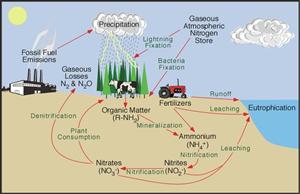
Dissolved inorganic forms of phosphorus include orthophosphate, PO4–3. Dissolved inorganic nitrogen is mainly present as nitrate, NO3–, and ammonium ion, NH4+, but also as dissolved nitrogen gas, N2; nitrite, NO2–; nitrous oxide, N2O; and ammonia, NH3.
The availability of nutrients for uptake and use by living organisms depends on the chemical form of the nutrient and the biochemical processes available to the organism. Some blue-green algae and bacteria can utilize nitrogen gas (N2), converting it to organic nitrogen—a process known as nitrogen fixation. Other bacteria, fungi, and plants use ammonium, and most can use nitrate. Phytoplankton and most plants assimilate orthophosphate, although some can access dissolved organic phosphorus using phosphatase enzymes. Particulate organic forms of phosphorus and nitrogen are slowly converted back into soluble forms.
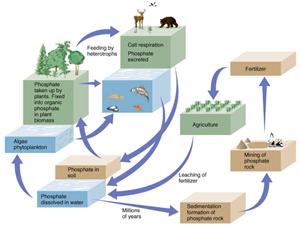
Sources of Nutrients
Phosphorus can be released from minerals through weathering, which is highly dependent on climate. Some inorganic materials in soils can bind phosphorus, preventing it from moving from terrestrial sources to the aquatic ecosystem. Artificial sources of nutrients include discharges of sewage, animal waste, and agricultural fertilizers. Direct discharge of wastewater or runoff can lead to elevated concentrations of nutrients in aquatic ecosystems. Because nutrient concentrations often limit primary productivity and constrain biomass, inputs of nutrients can lead to large increases in the growth of algae or aquatic plants, just as fertilization can increase growth and yield of crops.
Eutrophication
Eutrophication results when high concentrations of nutrients lead to excessive biological growth. While eutrophication is a natural process in the aging of some freshwater ecosystems, artificial (human-caused) eutrophication can degrade water quality and threaten desirable aquatic species. High levels of algae can decrease the penetration of sunlight through the water, preventing photosynthesis by submerged plants. Algae associated with eutrophication may release compounds with bad odours, bad tastes, or toxins. In addition, the death and decay of algae can lead to decreased concentrations of dissolved oxygen. These changes can affect the diversity of plants, animals, and other aquatic organisms, and interfere with human uses of the water.